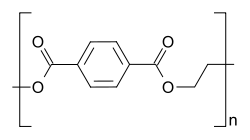
Uses
Plastic bottles made from PET are widely used for soft drinks (see carbonation). For certain specialty bottles, such as those designated for beer containment, PET sandwiches an additional polyvinyl alcohol (PVOH) layer to further reduce its oxygen permeability.
Biaxially oriented PET film (often known by one of its trade names, "Mylar") can be aluminized by evaporating a thin film of metal onto it to reduce its permeability, and to make it reflective and opaque (MPET). These properties are useful in many applications, including flexible food packaging and thermal insulation (such as space blankets). Because of its high mechanical strength, PET film is often used in tape applications, such as the carrier for magnetic tape or backing for pressure-sensitive adhesive tapes.
Non-oriented PET sheet can be thermoformed to make packaging trays and blister packs.[7] If crystallizable PET is used, the trays can be used for frozen dinners, since they withstand both freezing and oven baking temperatures. Both amorphous PET and BoPET are transparent to the naked eye. Color-conferring dyes can easily be formulated into PET sheet.
When filled with glass particles or fibres, it becomes significantly stiffer and more durable.
PET is also used as a substrate in thin film solar cells.
Terylene (a trademark formed by inversion of (polyeth)ylene ter(ephthalate)) is also spliced into bell rope tops to help prevent wear on the ropes as they pass through the ceiling.
PET is used since late 2014 as liner material in type IV composite high pressure gas cylinders. PET works as a much better barrier to oxygen than earlier used (LD)PE.[8]
PET is used as a 3D printing filament, as well as in the 3D printing plastic PETG.
History
PET was patented in 1941 by John Rex Whinfield, James Tennant Dickson and their employer the Calico Printers' Association of Manchester, England. E. I. DuPont de Nemours in Delaware, United States, first used the trademark Mylar in June 1951 and received registration of it in 1952.[9] It is still the best-known name used for polyester film. The current owner of the trademark is DuPont Teijin Films US, a partnership with a Japanese company.[10]
In the Soviet Union, PET was first manufactured in the laboratories of the Institute of High-Molecular Compounds of the USSR Academy of Sciences in 1949, and its name "Lavsan" is an acronym thereof (лаборатории Института высокомолекулярных соединений Академии наук СССР).[11]
The PET bottle was patented in 1973 by Nathaniel Wyeth.
Physical properties
PET in its natural state is a colorless, semi-crystalline resin. Based on how it is processed, PET can be semi-rigid to rigid, and it is very lightweight. It makes a good gas and fair moisture barrier, as well as a good barrier to alcohol (requires additional "barrier" treatment) and solvents. It is strong and impact-resistant. PET becomes white when exposed to chloroform and also certain other chemicals such as toluene.[13]
About 60% crystallization is the upper limit for commercial products[citation needed], with the exception of polyester fibers. Clear products can be produced by rapidly cooling molten polymer below Tg glass transition temperature to form an amorphous solid. Like glass, amorphous PET forms when its molecules are not given enough time to arrange themselves in an orderly, crystalline fashion as the melt is cooled. At room temperature the molecules are frozen in place, but, if enough heat energy is put back into them by heating above Tg, they begin to move again, allowing crystals to nucleate and grow. This procedure is known as solid-state crystallization.
When allowed to cool slowly, the molten polymer forms a more crystalline material. This material has spherulites containing many small crystallites when crystallized from an amorphous solid, rather than forming one large single crystal. Light tends to scatter as it crosses the boundaries between crystallites and the amorphous regions between them. This scattering means that crystalline PET is opaque and white in most cases. Fiber drawing is among the few industrial processes that produce a nearly single-crystal product.
Intrinsic viscosity
One of the most important characteristics of PET is referred to as intrinsic viscosity (IV).[14]
The intrinsic viscosity of the material, found by extrapolating to zero concentration of relative viscosity to concentration which is measured in deciliters per gram (dℓ/g). Intrinsic viscosity is dependent upon the length of its polymer chains but has no units due to being extrapolated to zero concentration. The longer the polymer chains the more entanglements between chains and therefore the higher the viscosity. The average chain length of a particular batch of resin can be controlled during polycondensation.
The intrinsic viscosity range of PET:[15]
Fiber grade:
- 0.40–0.70 Textile
- 0.72–0.98 Technical, tire cord
Film grade:
- 0.60–0.70 BoPET (biaxially oriented PET film)
- 0.70–1.00 Sheet grade for thermoforming
Bottle grade:
- 0.70–0.78 Water bottles (flat)
- 0.78–0.85 Carbonated soft drink grade
Monofilament, engineering plastic
- 1.00–2.00
Drying
PET is hygroscopic, meaning that it absorbs water from its surroundings. However, when this "damp" PET is then heated, the water hydrolyzes the PET, decreasing its resilience. Thus, before the resin can be processed in a molding machine, it must be dried. Drying is achieved through the use of a desiccant or dryers before the PET is fed into the processing equipment.
Inside the dryer, hot dry air is pumped into the bottom of the hopper containing the resin so that it flows up through the pellets, removing moisture on its way. The hot wet air leaves the top of the hopper and is first run through an after-cooler, because it is easier to remove moisture from cold air than hot air. The resulting cool wet air is then passed through a desiccant bed. Finally, the cool dry air leaving the desiccant bed is re-heated in a process heater and sent back through the same processes in a closed loop. Typically, residual moisture levels in the resin must be less than 50 parts per million (parts of water per million parts of resin, by weight) before processing. Dryer residence time should not be shorter than about four hours. This is because drying the material in less than 4 hours would require a temperature above 160 °C, at which level hydrolysis would begin inside the pellets before they could be dried out.
PET can also be dried in compressed air resin dryers. Compressed air dryers do not reuse drying air. Dry, heated compressed air is circulated through the PET pellets as in the desiccant dryer, then released to the atmosphere.
Copolymers
In addition to pure (homopolymer) PET, PET modified by copolymerization is also available.
In some cases, the modified properties of copolymer are more desirable for a particular application. For example, cyclohexane dimethanol (CHDM) can be added to the polymer backbone in place of ethylene glycol. Since this building block is much larger (6 additional carbon atoms) than the ethylene glycol unit it replaces, it does not fit in with the neighboring chains the way an ethylene glycol unit would. This interferes with crystallization and lowers the polymer's melting temperature. In general, such PET is known as PETG or PET-G (Polyethylene terephthalate glycol-modified; Eastman Chemical, SK Chemicals Selenis are some PETG manufacturers). PETG is a clear amorphous thermoplastic that can be injection molded, sheet extruded or extruded as filament for 3D printing. It can be colored during processing.
Another common modifier is isophthalic acid, replacing some of the 1,4-(para-) linked terephthalate units. The 1,2-(ortho-) or 1,3-(meta-) linkage produces an angle in the chain, which also disturbs crystallinity.
Such copolymers are advantageous for certain molding applications, such as thermoforming, which is used for example to make tray or blister packaging from co-PET film, or amorphous PET sheet (A-PET/PETA) or PETG sheet. On the other hand, crystallization is important in other applications where mechanical and dimensional stability are important, such as seat belts. For PET bottles, the use of small amounts of isophthalic acid, CHDM, diethylene glycol (DEG) or other comonomers can be useful: if only small amounts of comonomers are used, crystallization is slowed but not prevented entirely. As a result, bottles are obtainable via stretch blow molding ("SBM"), which are both clear and crystalline enough to be an adequate barrier to aromas and even gases, such as carbon dioxide in carbonated beverages.
Production
Polyethylene terephthalate is produced from ethylene glycol and dimethyl terephthalate(DMT) (C6H4(CO2CH3)2) or terephthalic acid.[16]
The former is a transesterification reaction, whereas the latter is an esterification reaction.
Dimethyl terephthalate process (DMT)
In dimethyl terephthalate(DMT) process, this compound and excess ethylene glycol are reacted in the melt at 150–200 °C with a basic catalyst. Methanol(CH3OH) is removed by distillation to drive the reaction forward. Excess ethylene glycol is distilled off at higher temperature with the aid of vacuum. The second transesterification step proceeds at 270–280 °C, with continuous distillation of ethylene glycol as well.[16]
The reactions are idealized as follows:
- First step
- C6H4(CO2CH3)2 + 2 HOCH2CH2OH → C6H4(CO2CH2CH2OH)2 + 2 CH3OH
- Second step
- n C6H4(CO2CH2CH2OH)2 → [(CO)C6H4(CO2CH2CH2O)]n + n HOCH2CH2OH
Terephthalic acid process
In the terephthalic acid process, esterification of ethylene glycol and terephthalic acid is conducted directly at moderate pressure (2.7–5.5 bar) and high temperature (220–260 °C). Water is eliminated in the reaction, and it is also continuously removed by distillation:[16]
- n C6H4(CO2H)2 + n HOCH2CH2OH → [(CO)C6H4(CO2CH2CH2O)]n + 2n H2O
Degradation
PET is subjected to various types of degradations during processing. The main degradations that can occur are hydrolytic, and probably most important, thermal oxidation. When PET degrades, several things happen: discoloration, chain scissions resulting in reduced molecular weight, formation of acetaldehyde, and cross-links ("gel" or "fish-eye" formation). Discoloration is due to the formation of various chromophoric systems following prolonged thermal treatment at elevated temperatures. This becomes a problem when the optical requirements of the polymer are very high, such as in packaging applications. The thermal and thermooxidative degradation results in poor processibility characteristics and performance of the material.
One way to alleviate this is to use a copolymer. Comonomers such as CHDM or isophthalic acid lower the melting temperature and reduce the degree of crystallinity of PET (especially important when the material is used for bottle manufacturing). Thus, the resin can be plastically formed at lower temperatures and/or with lower force. This helps to prevent degradation, reducing the acetaldehyde content of the finished product to an acceptable (that is, unnoticeable) level. See copolymers, above. Another way to improve the stability of the polymer is to use stabilizers, mainly antioxidants such as phosphites. Recently, molecular level stabilization of the material using nanostructured chemicals has also been considered.
Acetaldehyde
Acetaldehyde is a colorless, volatile substance with a fruity smell. Although it forms naturally in some fruit, it can cause an off-taste in bottled water. Acetaldehyde forms by degradation of PET through the mishandling of the material. High temperatures (PET decomposes above 300 °C or 570 °F), high pressures, extruder speeds (excessive shear flow raises temperature), and long barrel residence times all contribute to the production of acetaldehyde. When acetaldehyde is produced, some of it remains dissolved in the walls of a container and then diffuses into the product stored inside, altering the taste and aroma. This is not such a problem for non-consumables (such as shampoo), for fruit juices (which already contain acetaldehyde), or for strong-tasting drinks like soft drinks. For bottled water, however, low acetaldehyde content is quite important, because, if nothing masks the aroma, even extremely low concentrations (10–20 parts per billion in the water) of acetaldehyde can produce an off-taste.
Antimony
Antimony (Sb) is a metalloid element that is used as a catalyst in the form of compounds such as antimony trioxide (Sb2O3) or antimony triacetate in the production of PET. After manufacturing, a detectable amount of antimony can be found on the surface of the product. This residue can be removed with washing. Antimony also remains in the material itself and can, thus, migrate out into food and drinks. Exposing PET to boiling or microwaving can increase the levels of antimony significantly, possibly above USEPA maximum contamination levels.[17] The drinking water limit assessed by WHO is 20 parts per billion (WHO, 2003), and the drinking water limit in the United States is 6 parts per billion.[18] Although antimony trioxide is of low toxicity when taken orally,[19]its presence is still of concern. The Swiss Federal Office of Public Health investigated the amount of antimony migration, comparing waters bottled in PET and glass: The antimony concentrations of the water in PET bottles were higher, but still well below the allowed maximum concentration. The Swiss Federal Office of Public Health concluded that small amounts of antimony migrate from the PET into bottled water, but that the health risk of the resulting low concentrations is negligible (1% of the "tolerable daily intake" determined by the WHO). A later (2006) but more widely publicized study found similar amounts of antimony in water in PET bottles.[20] The WHO has published a risk assessment for antimony in drinking water.[19]
Fruit juice concentrates (for which no guidelines are established), however, that were produced and bottled in PET in the UK were found to contain up to 44.7 µg/L of antimony, well above the EU limits for tap water of 5 µg/L.[21][22]
Biodegradation
At least one species of bacterium in the genus Nocardia can degrade PET with an esterase enzyme.[23]
Japanese scientists have isolated a bacterium Ideonella sakaiensis that possesses two enzymes which can break down the PET into smaller pieces that the bacterium can digest. A colony of I. sakaiensis can disintegrate a plastic film in about six weeks.[24][25]
Safety
Commentary published in Environmental Health Perspectives in April 2010 suggested that PET might yield endocrine disruptors under conditions of common use and recommended research on this topic.[26] Proposed mechanisms include leaching of phthalates as well as leaching of antimony. An article published in Journal of Environmental Monitoring in April 2012 concludes that antimony concentration in deionized water stored in PET bottles stays within EU's acceptable limit even if stored briefly at temperatures up to 60 °C (140 °F), while bottled contents (water or soft drinks) may occasionally exceed the EU limit after less than a year of storage at room temperature.
Bottle processing equipment
There are two basic molding methods for PET bottles, one-step and two-step. In two-step molding, two separate machines are used. The first machine injection molds the preform, which resembles a test tube, with the bottle-cap threads already molded into place. The body of the tube is significantly thicker, as it will be inflated into its final shape in the second step using stretch blow molding.
In the second step, the preforms are heated rapidly and then inflated against a two-part mold to form them into the final shape of the bottle. Preforms (uninflated bottles) are now also used as robust and unique containers themselves; besides novelty candy, some Red Cross chapters distribute them as part of the Vial of Life program to homeowners to store medical history for emergency responders.
In one-step machines, the entire process from raw material to finished container is conducted within one machine, making it especially suitable for molding non-standard shapes (custom molding), including jars, flat oval, flask shapes, etc. Its greatest merit is the reduction in space, product handling and energy, and far higher visual quality than can be achieved by the two-step system.
| Names | |
|---|---|
| IUPAC name
Poly(ethyl benzene-1,4-dicarboxylate)
|
|
| Identifiers | |
|
CAS Number
|
|
| Abbreviations | PET, PETE |
| ChemSpider |
|
| ECHA InfoCard | 100.121.858 |
| Properties | |
|
Chemical formula
|
(C10H8O4)n[1] |
| Molar mass | variable |
| Density |
1.38 g/cm3 (20 °C),[2] amorphous: 1.370 g/cm3, [1]single crystal: 1.455 g/cm3[1] |
| Melting point |
> 250 °C (482 °F; 523 K) [2]260 °C[1] |
| Boiling point |
> 350 °C (662 °F; 623 K) (decomposes) |
|
Solubility in water
|
practically insoluble[2] |
| log P | 0.94540[3] |
| Thermal conductivity |
0.15[4] to 0.24 W m−1 K−1[1] |
|
Refractive index(nD)
|
1.57–1.58,[4] 1.5750[1] |
| Thermochemistry | |
|
Heat capacity (C)
|
1.0 kJ/(kg·K)[1] |
| Related compounds | |
|
Related Monomers
|
Terephthalic acid Ethylene glycol |
|
Except where otherwise
noted, data are given for
materials in their standard
state (at 25 °C [77 °F], 100 kPa).
|
|